
News & Events

News & Events
Recently, a research paper entitled "Membrane electrode assembly design for lithium-mediated electrochemical nitrogen reduction" was published in Energy & Environmental Science (IF = 39.714), which is a well-known international journal in the field of energy and environment. In this paper, a feasible membrane electrode assembly (MEA) configuration is proposed for lithium-mediated electrochemical nitrogen reduction to produce ammonia. The first author is Xiyang Cai, a doctoral student at Institute of Fuel Cells of School of Mechanical Engineering, Shanghai Jiao Tong University, and the corresponding authors are Prof. Junliang Zhang and Prof. Shuiyun Shen.
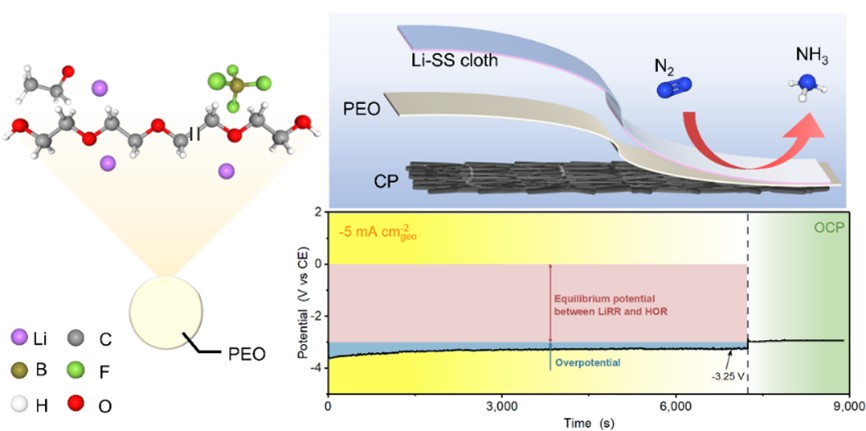
Schematic of membrane electrode assembly its performance toward lithium-mediated nitrogen reduction
As an important feedstock, ammonia is closely related to human society. Since the early twentieth century, artificial ammonia production has predominantly relied on the Haber–Bosch reaction coupled with steam reforming process, resulting in high energy consumption and high carbon dioxide emissions. In the context of energy conservation and decarbonization, lithium-mediated electrochemical nitrogen reduction reaction (LiNR) is regarded as a promising alternative to the Haber–Bosch process but is still hampered by its poor gas transfer, dependence on organic solvent and significant voltage loss.
In this work, the research team firstly demonstrated the possibility of realizing LiNR by MEA configuration. The MEA was composed of lithium deposited stainless-steel cloth at cathode, lithium doped polyethylene oxide (PEO) as polymer electrolyte and carbon paper loaded with Pt/C catalysts at anode. A mean ammonia production rate of 2.41 ± 0.14 μmol h-1 cm-2geo and a faradaic efficiency of 8.9 ± 1.7% was obtained at cell voltage of 3.6 V. Lower voltage loss (ca. 0.25 V@5 mA cm-2geo) can be achieved in the absence of ethanol. In-situ X-ray diffraction (XRD) and ex-situ X-ray photoelectron spectroscopy (XPS) and time-of-flight secondary ion mass spectrometry (ToF-SIMS) measurements were employed to reveal the transformation of lithium deposits. This work offers a new route for LiNR by advantages of efficient gas transfer, reduced solvent consumption and compact configuration.
Paper Link: https://pubs.rsc.org/en/content/articlelanding/2023/ee/d3ee00026e
Source: School of Mechanical Engineering, Shanghai Jiao Tong University
Editor on Duty: Diwei Chen
Responsible Editor: Qianqian Jiang, Yuhe Fu

SCHOOL OF PHARMACY,SJTU
Address: 800 Dongchuan Road, Shanghai
200240